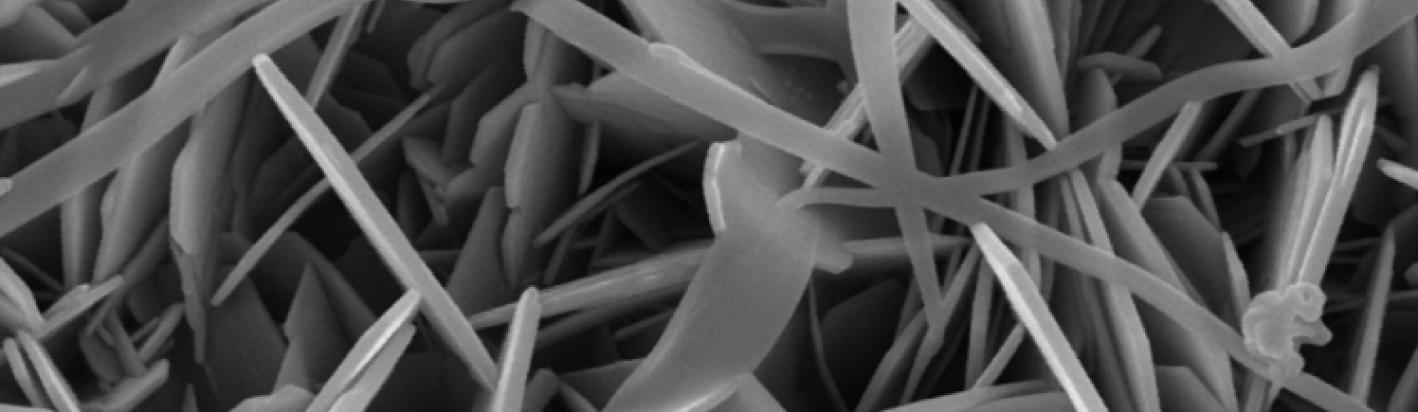
The most important members of the kaolin sub group of minerals are kaolinite and halloysite. Kaolinite has the formula Al2Si2O5(OH)4 and typically occurs in platy forms. Halloysite has a similar composition except that it contains additional water molecules between the layers and most commonly has a tubular morphology. Halloysite loses its interlayer water very easily so it is often observed in a partly dehydrated state. In its fully hydrated form the formula is Al2Si2O5(OH)4 -2H2O. Kaolinite is a very important industrial mineral, and halloysite is becoming increasingly important due mainly to its use in nanotechnology applications which take advantage of its tubular habit.
Kaolins and halloysites can be analysed by a variety of methods. Full pattern fitting of XRPD pattern obtained from spray dried powders is used to quantify kaolins and halloysites and can accurately determine the proportions of both kaolinite and halloysite when they are present in the same sample - along with any other minerals that are present. Infrared spectroscopy is also particularly sensitive to the different kaolin polytypes and provides a variety of information that is complimentary to that obtained by XRPD. Imaging by SEM provides information on particle size, form and association. Other methods such as identification of halloysite by formamide intercalation can also be performed and with analysis of clay sized fractions the response to solvation with ethylene glycol is also diagnostic of halloysite (Hillier and Ryan 2002).
- Hillier, S., and P. C. Ryan, 2002, Identification of halloysite (7 angstrom) by ethylene glycol solvation: the 'MacEwan effect': Clay Minerals, v. 37, no. 3, p. 487-496. PDF